Address
3633 Central Ave. Hot Springs, AR 71913
Phone
(501) 620-4449
Antimicrobial resistance is given the acronym AMR. It is not something most practitioners think of daily, but is a global public health threat. According to the World Health Organization, each year 1.27 million deaths result from AMR. The cause is attributed to the misuse and overuse of antibiotics in humans, plants, and animals. Every country is affected, but the low and middle-income countries are most affected. Due to resistance to antibiotics, infections have become harder to treat. Many skin infections caused by staphylococcus aureus have become resistant to commonly used antibiotics. While there is an urgent need for new antibiotics, drug companies are hesitant to invest their dollars in the new antibiotic pipeline.
Binary Pharmaceuticals believes using common antibiotics judiciously is a step in the right direction to combat AMR. The amount of antibiotics consumed globally for surgical antibiotic prophylaxis is enormous, representing 15% of the world’s use of antibiotics. The Binary Platform incorporates microdoses of antibiotics without the impact to the gut microbiome seen with oral antibiotics. The degree to which cutaneous microdosing affects the human microbiome is unknown. The effects of oral antibiotics and subsequent antibiotic scarring are well documented. The Binary platform has the opportunity to limit the degree of dysbiosis and damage to the microbiome. Antibiotic toxicities should be minimized, and the proven benefits of surgical prophylaxis with this delivery technique have been proven in clinical trials.
The W.H.O. Fact sheet on Antimicrobial Resistance (AMR) is widely quoted.
In the last ten years, it has become apparent that commonly prescribed antibiotics have persistent long-term effects on the gut microbiome. One well-known expert, Dr. Shaefer Spires from the Duke Center for Antimicrobial Stewardship and Infection Prevention, states: “Even short courses of antibiotics have acute disturbances in the gut microbiome and may take months to recover. Long story short, it’s worse than we thought.”1
Antibiotics decrease the microbiome’s overall bacteria load. In one study, 20 volunteers were recruited and divided into four groups. Each group received a different antibiotic. They found the overall composition and resistome did not return to normal two months after discontinuation of antibiotics.1 Scientists use the term resistance elements to test and identify the changes in the resistant gene burden. It was found this burden increased significantly for those who had received antibiotics. There are also long-term consequences in the composition of the gut microbiome, even when the overall microbial burden recovers. These resistant elements increase and become incorporated into the gut microbiome. Antibiotic scarring refers to long-term permanent changes in the gut microbiome due to antibiotics.
A simplified explanation of how antibiotics affect the gut flora, is that they disrupt microbial diversity. They may deplete beneficial species of bacteria while allowing opportunistic bacteria to thrive, leading to an imbalance called dysbiosis. When the beneficial bacteria are suppressed, pathogens like Clostridiodes difficile can overgrow, leading to colitis and persistent infections.
Even slight microbial disruptions can lead to an imbalance. The gut microbiome assists in digesting fiber, producing vitamins, and modulating the immune system. Any disruption can lead to infections and inflammatory disease.
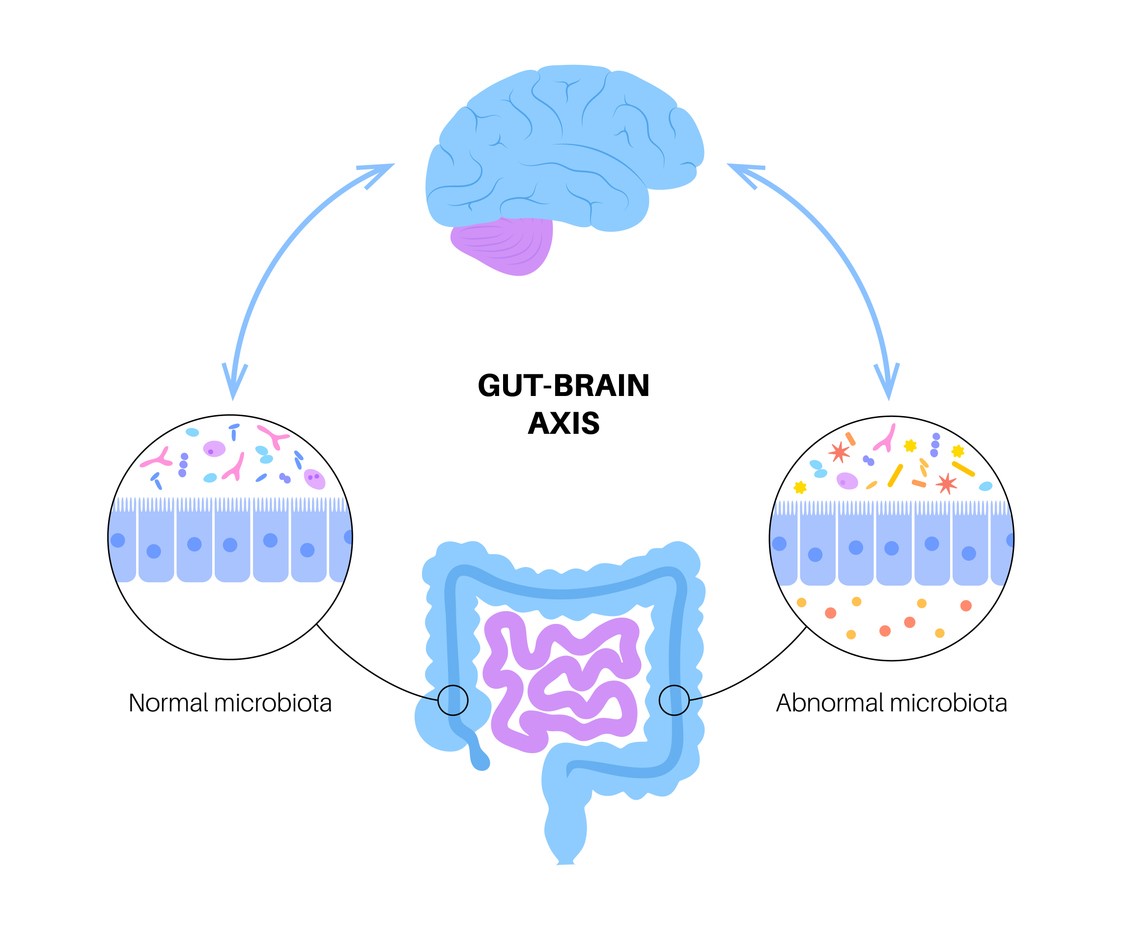
After a short course of antibiotics, the gut microbiome can take weeks or months to recover. Some species may never return, particularly after a prolonged course of antibiotics. Antibiotic exposure in early life has been linked to an increase in obesity, allergies, and autoimmune disease. The exact strength of this linkage is not known, but neonates, obese children, and children with allergic rhinitis compromise a vulnerable population for antibiotic-associated dysbiosis.
Clinical trials have shown that probiotics can help to some extent and improve H pylori eradication rates. All providers need to consider the deleterious effects of antibiotics on the microbiome before prescribing. The prudent use of antimicrobials can help prevent gut microbial dysbiosis.2
Proposed Work and Milestones
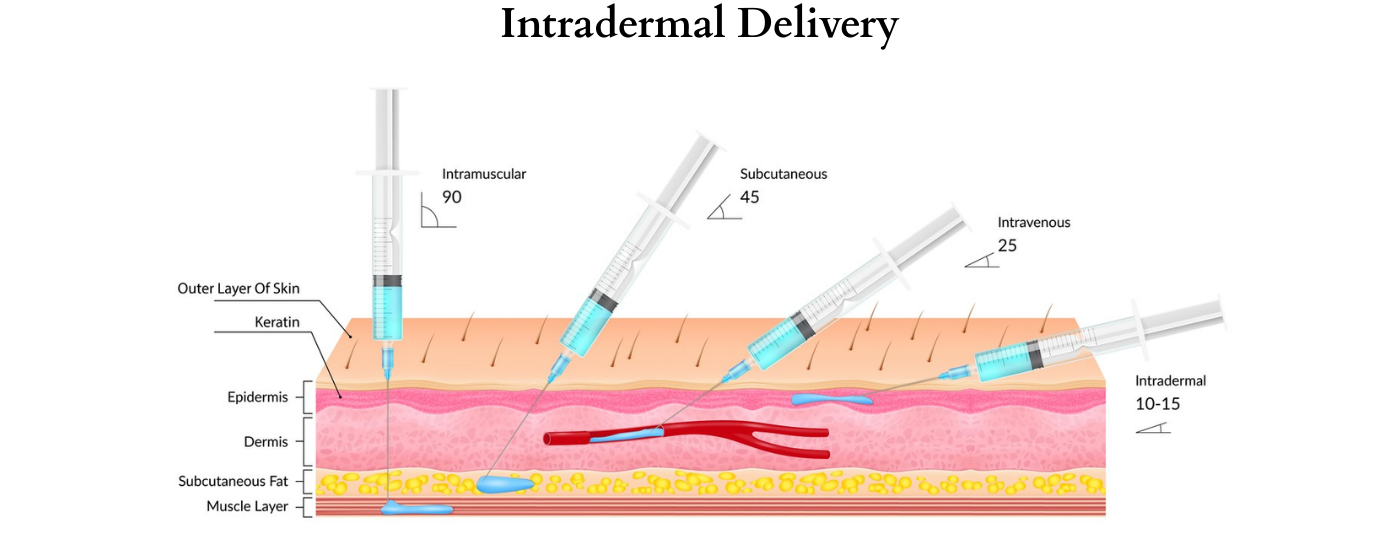
Our proposed plan to implement our technology is a strategic 60-month program organized into three distinct yet interconnected phases. This timeline aims to quickly progress from trial to final platform and worldwide adoption. Each phase has specific objectives and is associated with a measurable midterm milestone, ensuring accountability and a clear path to establishing intradermal delivery as the new global standard of care.
1. Phase 1 (0-24 months): Platform Optimization
Initial phase focusing on finalizing the target product through a preclinical study on optimizing syringe concentrations while minimizing systemic absorption. In addition, 24-month room temperature stability testing. The pH of the prefilled syringe will be optimized to a range of 6.7 -7.0 to minimize discomfort. Onshore CDMOs will perform the work. The scope of the work has been defined, and cost estimates received.
2. Phase 2 (24-48 months): Validation.
Building on the success of Phase 1, we will start large-scale trials in cutaneous surgery. We will pursue a 505(b)(2) NDA pathway through the FDA. Although all drugs targeted for Phase 2 are generic, they require clinical trials due to the use of a novel intradermal delivery method. While other drugs are approved for administration via this route, no antibiotics are approved through this delivery method. The data collected will help validate and refine our model, which predicts the long-term impact on the prevalence of resistant bacteria.
A Phase 2 milestone will be the reduction in surgical site infections in the active arm versus the placebo arm.
3. Phase 3 (48-60 months): Global Scaling
The final phase concentrates on translating our conclusive evidence into global policy and practice by developing comprehensive implementation packages, including training materials, protocols, and economic models for healthcare systems worldwide. A primary activity will involve formal engagement with the World Health Organization (WHO), GARD-P, the Gates Foundation, the Clinton Health Access Initiative, Surgical Infection Society, the Society for Healthcare Epidemiology of America, and prominent professional societies in the fields of general surgery and infectious disease. We will present the evidence and advocate for the revision of clinical practice guidelines by the American Society of Health System Pharmacist (ASHP). We have contacted the societies above and presented them with the plan. We have obtained endorsements from most of the organizations referenced as well. Discussions have been held with GARD-P, the Gates Foundation, and the Wellcome Trust.
Midterm Milestone: Formal integration of this prophylactic method into primary international clinical practice guidelines. Several authors have previously advocated for amendments to the published guidelines (ASHP) regarding surgical antibiotic prophylaxis. This will serve as a crucial factor in the global acceptance and adaptation of this innovative delivery system.
Incidence, Economic Impact, and Statistical Burden of Surgical Site Infections
Despite advances in surgical protocols and sterilization techniques, the incidence of surgical site infections (SSIs) has not decreased significantly and has remained stable for over a decade. Recent data indicate that progress in reducing SSI rates has stalled, with some measures showing rates have plateaued or even worsened in recent years. For example, a 2023 analysis of Centers for Disease Control and Prevention (CDC) data found a significant 3% increase in the standardized infection ratio (SIR) for operative procedure categories compared with the previous year. Research evaluating the Centers for Medicare & Medicaid Services (CMS) Hospital-Acquired Condition Reduction Program found no evidence of a decreasing trend in SSIs for targeted procedures such as abdominal hysterectomy and colon surgeries over the study period, further suggesting that traditional prevention measures may have reached an efficacy Ceiling.
Currently, it is estimated that approximately 400,000 to 500,000 SSIs occur annually in the United States. The economic burden of these infections is substantial, costing the U.S. healthcare system an estimated $3.3 billion to $10 billion annually. The cost of a single SSI varies widely by severity and procedure type; while the average cost is often cited as exceeding $20,000 per admission, it can range from approximately $10,497 for general and vascular surgeries to over $90,000 if the infection involves a prosthetic implant. Detailed analyses of specific specialties reveal that infections following neurosurgery are the costliest to treat (attributable cost of $23,755), followed by orthopedic surgery ($15,243). Additionally, deep-incisional SSIs following spinal fusion have been shown to incur incremental payments ranging from $48,662 to $93,741 over a 24-month period. Beyond direct treatment costs, SSIs drive significant use of healthcare resources through readmissions and extended hospital stays. Patients with an SSI are up to five times more likely to be readmitted to the hospital and twice as likely to die as non- infected patients. The cost of an SSI-related readmission is high; for Medicare patients, the mean cost of a readmission for a non-infectious surgical site complication is estimated at $21,478, while readmissions for SSIs specifically can average $23,136. Furthermore, SSIs prolong hospital length of stay (LOS) by a median of 7 to 11 days, representing a substantial opportunity cost for hospital bed capacity.
Our innovation represents a shift in preventing surgical infections, moving from an inefficient, systemic approach to a precise, evidence-based methodology. Implementation will deliver substantial and synergistic benefits across clinical, economic, and global public health sectors by directly addressing the intertwined crises of surgical site infections (SSIs) and antimicrobial resistance (AMR).
The intradermal/intra-incisional delivery platform is simple but will have profound effects. It is not a new drug but a superior method of deploying trusted generic antibiotics. Our strategy includes:
• Precision Targeting: By delivering antibiotics directly to the incision site, the platform neutralizes pathogens at the source before contaminating the surgical wound. Delivery during, what is known as, the “Golden Hour” contrasts sharply with the systemic approach, which often delivers sub-therapeutic doses to the target tissue too late.
• Technological Simplicity: This method can be implemented immediately in hospitals, outpatient, and clinic environments. Since this does not involve fancy technology, the scalability and applicability to healthcare settings with strained resources and austere environments are not burdened.
• Global Accessibility: Our platform depends on affordable, widely accessible generic antibiotics such as clindamycin and cefazolin. There are four to six commonly utilized antibiotics that can be instantaneously adapted for intradermal and intralesional delivery. This practical and sustainable solution is suitable for high-, middle- and low-income nations, regardless of the intricate supply chains and elevated costs linked to novel pharmaceuticals.
• Platform Versatility: The fundamental principle is applicable across a vast range of procedures. It has demonstrated efficacy and can be adapted for protocols in general surgery, orthopedics, dermatological surgery, Ob/Gyn, cardiac surgery, ER, neurosurgery, oral surgery and more.
Decoupling from novel drug development involves circumventing the ineffective, financially imprudent pipeline for new antibiotics and significantly improving the efficacy of existing, cost-effective generic medications. This approach ensures a sustainable and globally accessible solution to SSIs and antimicrobial resistance (AMR).